Overview of Research Programs
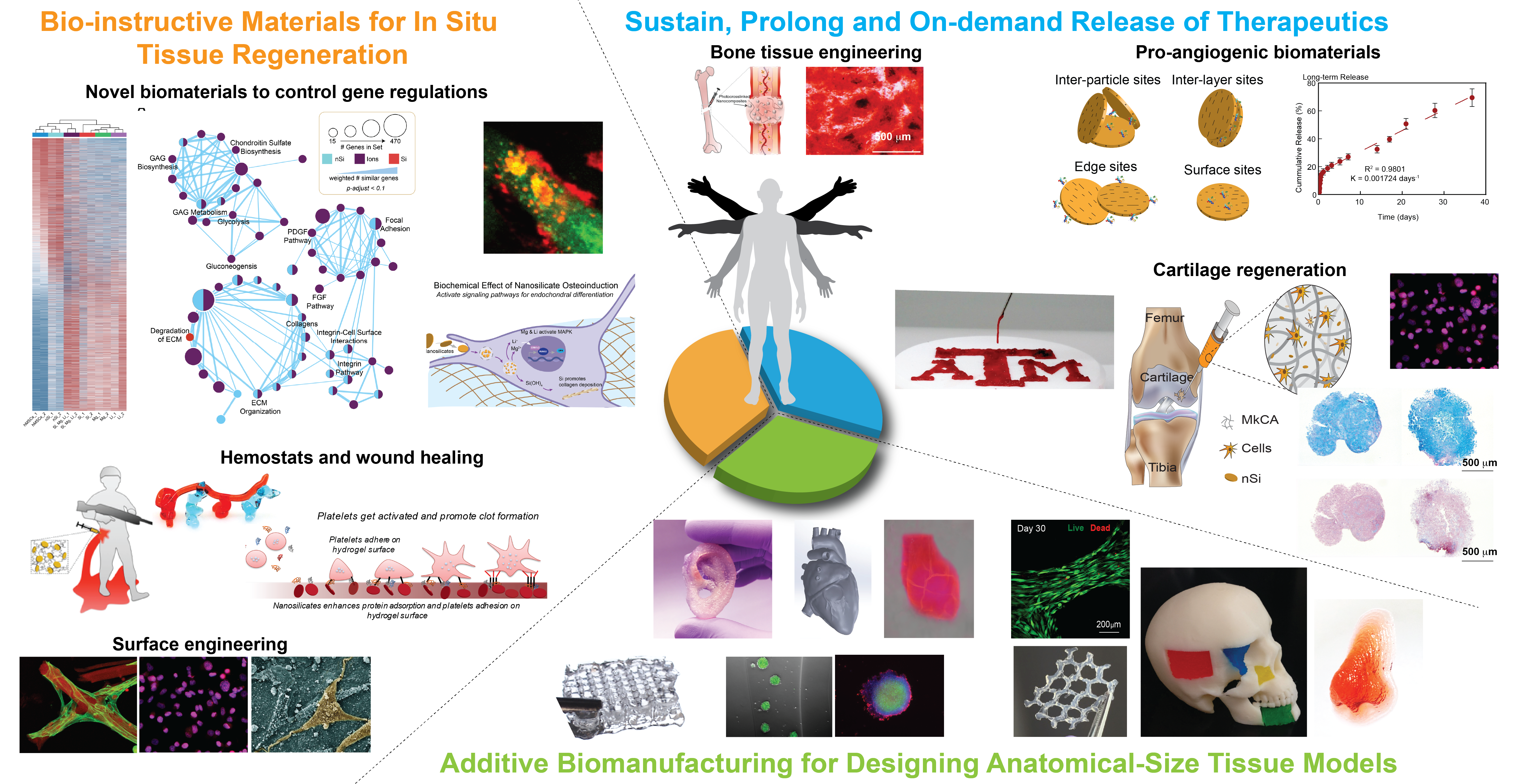
The goal of our lab is to design new biomaterials for in situ tissue regeneration, therapeutics delivery and additive biomanufacturing. In particular, our lab is leveraging principles from materials science, stem cell biology, bioengineering and high throughput genomics to design smart and responsive biomaterials, with wide-ranging applications in the field of regenerative medicine, and cancer biology. The three major area of our research as shown in the overview figure:
Bio-instructive Materials for In Situ Tissue Regeneration
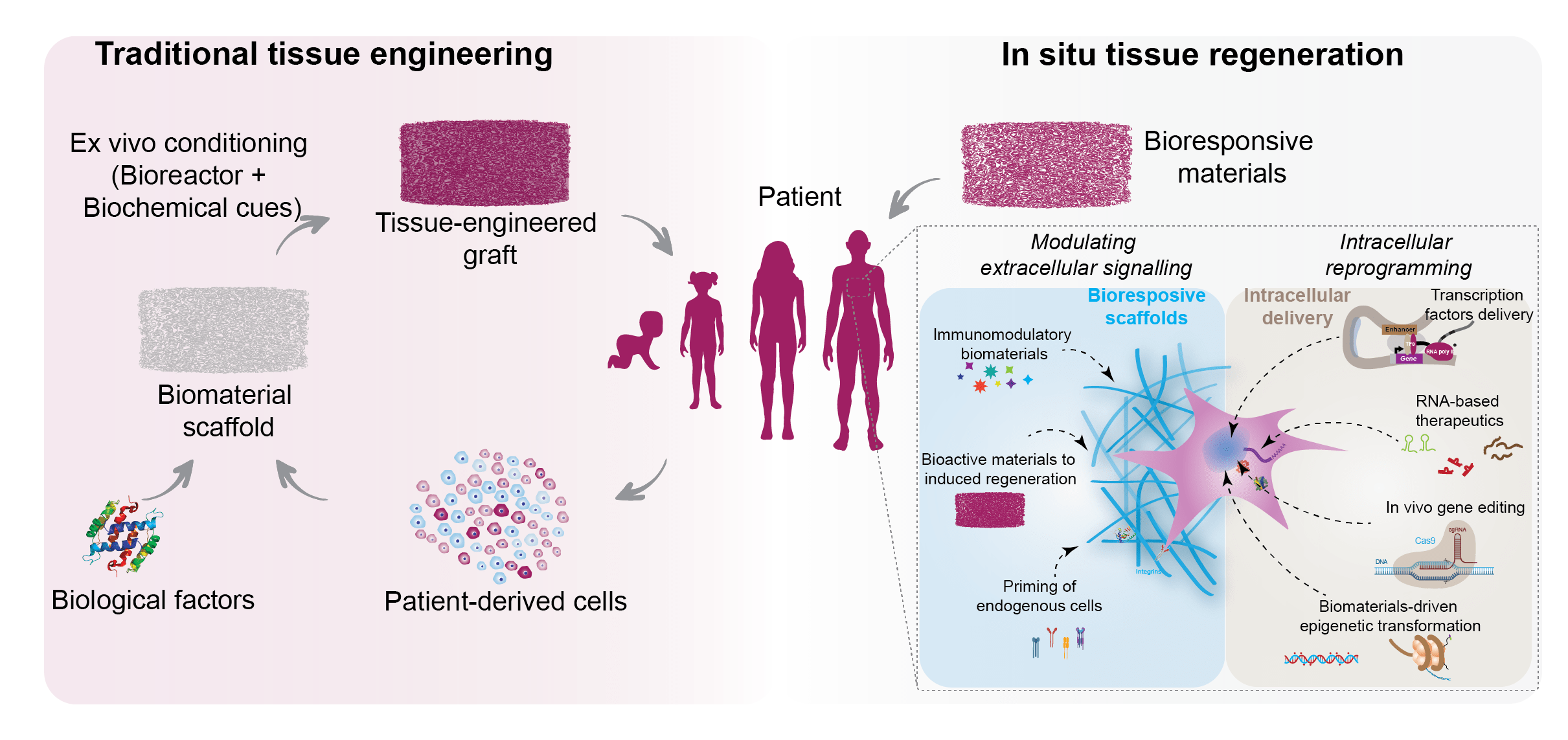
In situ tissue regeneration harnesses the body’s regenerative potential to control and direct cell functions for tissue repair. The design of biomaterials for in situ tissue engineering requires precise control over biophysical and biochemical cues to mobilize endogenous cells to the site of injury. Specifically, the native microenvironment needs to provide the necessary cues to direct host stem/progenitor cells to repopulate implanted, acellular scaffolds. These cues need to induce regeneration by modulating extracellular microenvironment or drive cellular reprogramming for in situ tissue repair.
In our lab, we design a range of bioresponsive materials to control and direct the body’s regenerative capacity for tissue-specific regeneration. Specifically, biomaterials loaded with bioactive cues that prime endogenous cells to perform tissue-specific functions is utilized. In addition, immune-interactive biomaterials to proactively modulate the inflammatory response towards tissue healing, integration, and regeneration is also investigated. In addition, we are also developing target-specific biomaterials for cellular reprogramming to stimulate the tissue regeneration process. We expect that leveraging the regenerative potential of the human body via the novel design of smart and responsive biomaterials provides a simple and effective approach to replace injured or diseased tissues.
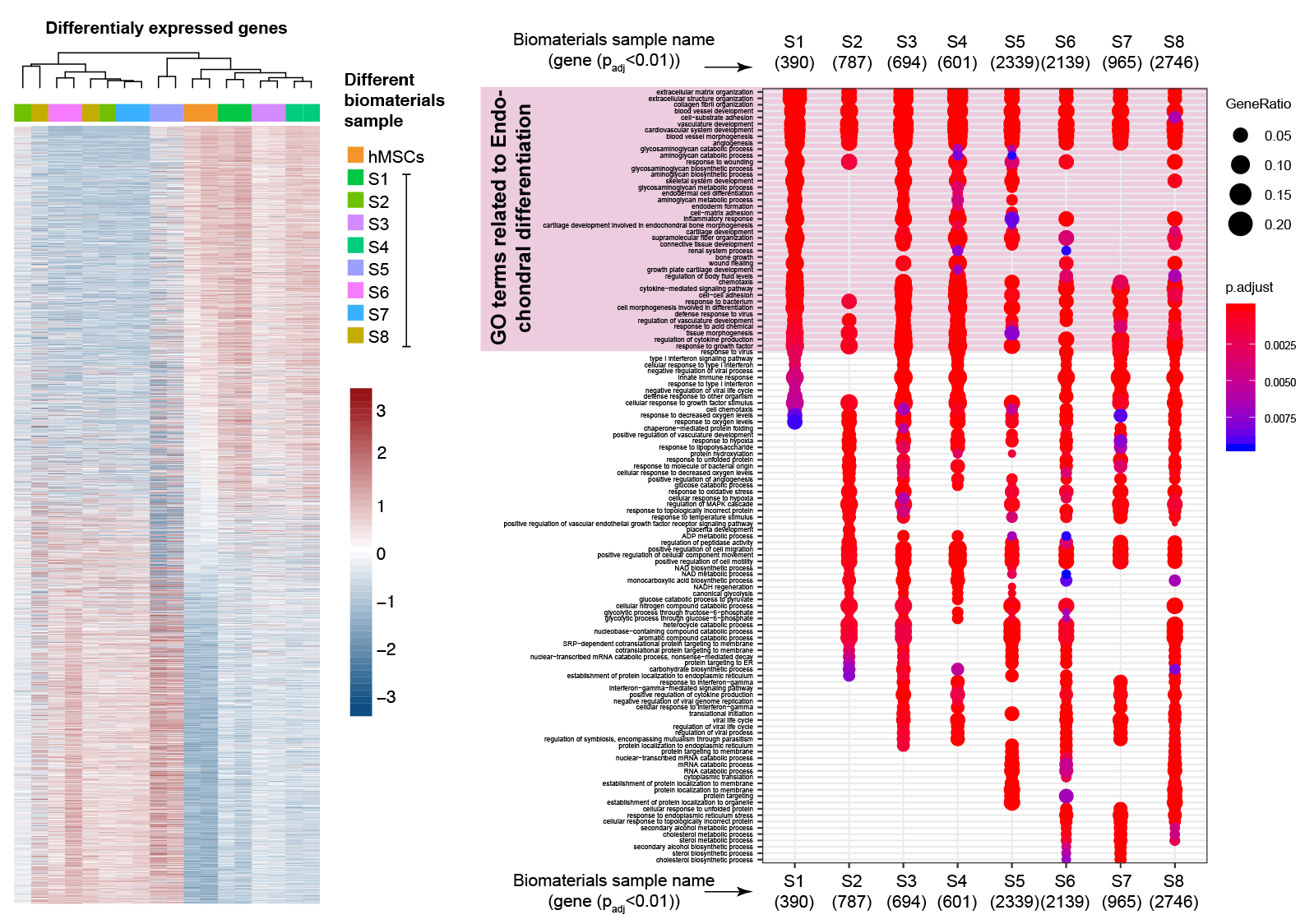
One of the core strenght of our collaborator (Dr. Irtisha Singh, Molecular and Cellular Medicine) is to leverage advances in biology to design next generation of bioinstructive materials. “Omics” techniques utilized by Dr. Singh is able to provid readouts of different biological states, and thus allow us to understand complex biological interactions of biomaterials and biomedical devices. Specifically, we are using various genome wide assays to capture information about changes in mRNA levels to assess changes in genomic accessibility that have laid down the necessary foundation to provide an unbiased global view of the cellular activity with pivotal insights about the affected cellular pathways.
Sustain, Prolong and On-demand Release of Therapeutics
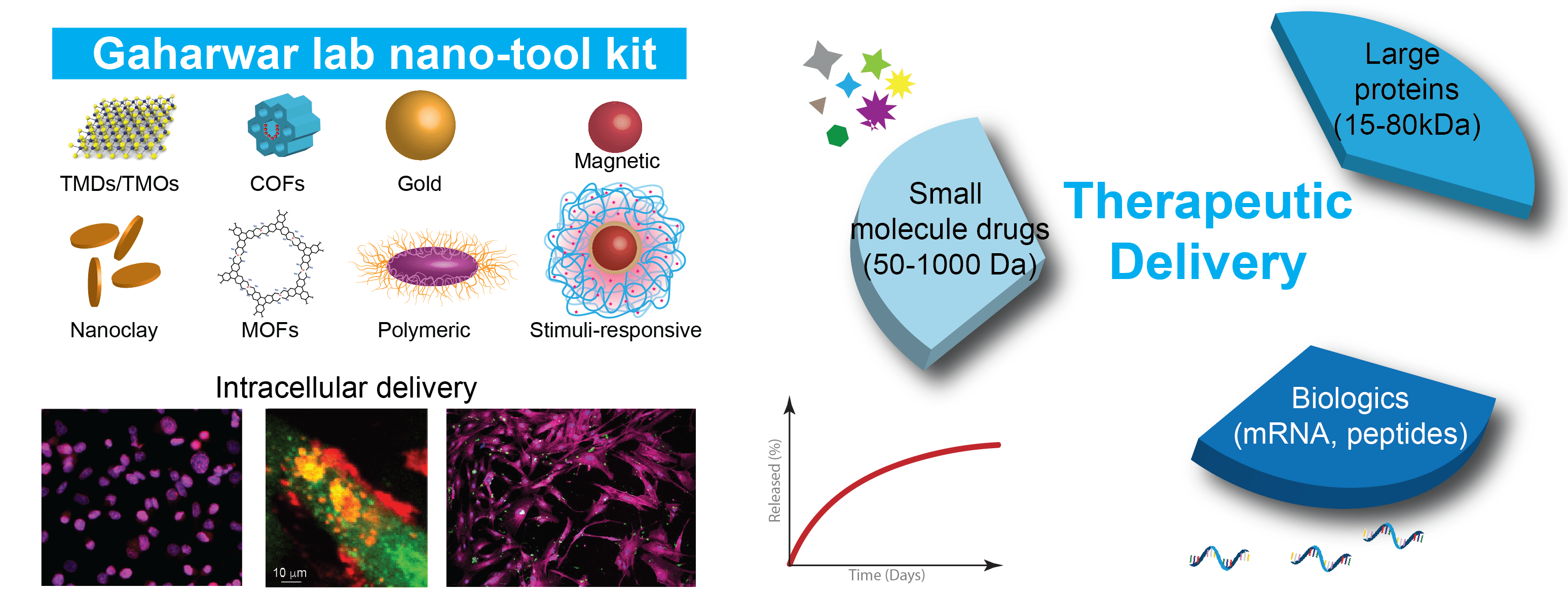
Intracellular delivery of therapeutics holds remarkable potential in regenerative medicine, stem cell engineering, immune modulation, and cancer therapeutics. However, direct delivery of therapeutics suffers from their in vitro and in vivo instability, immunogenicity, and a relatively short half-life within the body. To overcome these shortcomings, we have designed and developed a range of biomaterials to enhance therapeutics loading efficiency, reduce inflammatory responses, and control release kinetics. Specifically, we have developed a range of two-dimensional nanomaterials such as nanoclay (nanosilciates), covalent organic framework and transition metal dichalcogenides.
One of the works focused on developing anti-inflammatory and pro-regenerative nanoparticles for cartilage regeneration. More recently, we havedemonstrated that the release of therapeutics can be controlled by exposure to external or internal stimuli, such as light, enzymes, or pH. These studies have provided the proof-of-concept for designing the next generation of responsive materials for various biomedical applications. These nanoparticles can be incorporated in the hydrogels matrix and can be injected for local delivery of therapeutics and can be used for wound healing.
Additive Biomanufacturing of Anatomical-Size Tissue Models
Three-dimensional (3D) bioprinting is emerging as a promising method for rapid fabrication of biomimetic cell-laden constructs for tissue engineering using cell-containing hydrogels, called bioinks, that can be crosslinked to form a hydrated matrix for encapsulated cells. However, extrusion based 3D bioprinting has hit a bottleneck in progress due to the lack of available bioinks with high printability, mechanical strength, and biocompatibility. Our lab has introduced multiple approaches to design highly printable bioink for fabricating large scale, cell-laden, bioactive scaffolds. Specifically, we have introduced a range of bioink formulation consisting of nanoengineered bioinks, ionic-covalent entanglement (ICE) bioinks and nanoengineered ionic-covalent entanglement (NICE) bioinks with excellent printability, mechanical properties, and shape-fidelity.
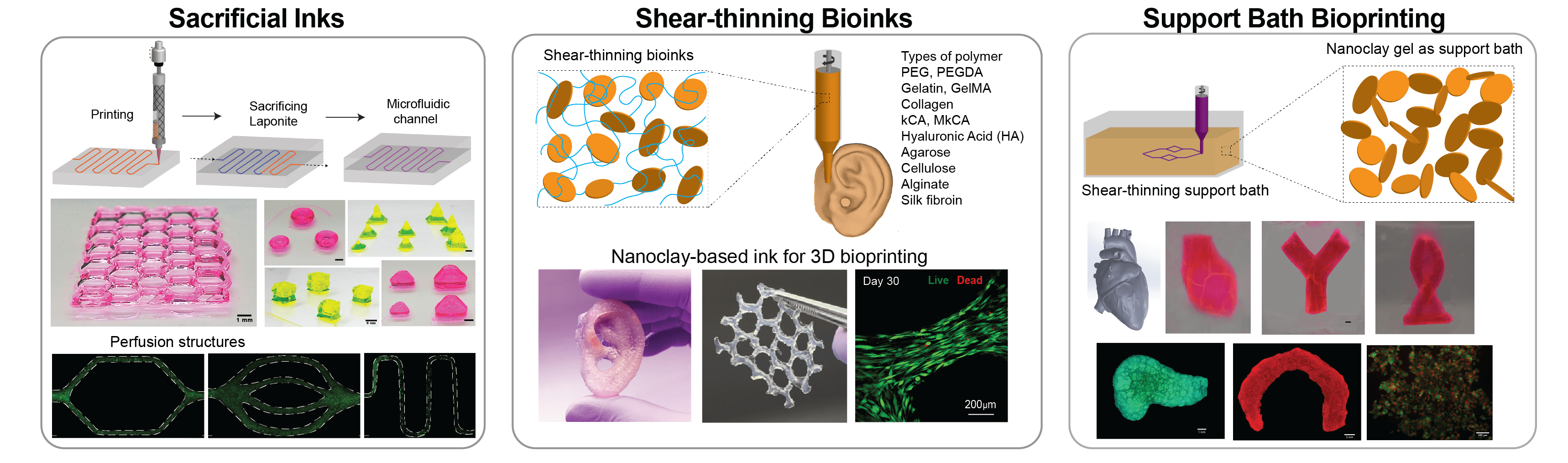
In addition, we have also introduced colloidal solutions of two-dimensional (2D) nanosilicates as a platform technology to print complex structures via three different approaches. In the first approach, we designed a shear-thinning ink composed of nanosilicates, which can be further reinforced by adding different types of water-soluble polymers. In the second approach, we demonstrated the use of nanosilicates as a sacrificial ink to design microfluidic devices for in vitro disease modelling. In the third approach, we utilized a colloidal nanosilicate gel as a support bath for 3D printing by nullifying the surface tension and gravitational forces. Due to their exceptional versatility, nanosilicate-based biomaterials could be widely adopted in the fields of additive manufacturing, tissue engineering, drug delivery, and medical devices.